40 fda approved drug labels
Generic Drugs - Specific Labeling Resources | FDA ANDA holders can also obtain the last approved RLD labeling from FDA's Division of Freedom of Information via online request, fax (301-827-9267), or mail (FDA Division of Freedom of Information... FDA Approves Mounjaro - drugs.com FDA Approves Mounjaro INDIANAPOLIS , May 13, 2022 /PRNewswire/ -- The U.S. Food and Drug Administration (FDA) approved Mounjaro (tirzepatide) injection, Eli Lilly and Company's (NYSE: LLY) new once-weekly GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptor agonist indicated as an adjunct to diet and ...
Drug Safety-related Labeling Changes (SrLC) ( TRIPTORELIN PAMOATE) Safety-related Labeling Changes Approved by FDA Center for Drug Evaluation and Research (CDER) Download Data Expand all 04/26/2022 (SUPPL-10) Questions related to the drug...

Fda approved drug labels
FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents.... Drugs@FDA: FDA-Approved Drugs This report displays final approvals and tentative approvals of original and supplemental applications for the two weeks beginning on the earliest date listed below. Some approvals may be added to...
Fda approved drug labels. Zuranolone FDA Approval Status - Drugs.com FDA Approved: No. Generic name: zuranolone. Company: Sage Therapeutics, Inc. Treatment for: Major Depressive Disorder, Postpartum Depression. Zuranolone is an investigational oral neuroactive steroid (NAS) GABA-A receptor positive allosteric modulator (PAM) in development for the treatment of major depressive disorder (MDD) and postpartum ... Animal Drug Safety-Related Labeling Changes | FDA Prascend (pergolide tablets), NADA 141-331, August 6, 2021 Terramycin 100MR (oxytetracycline), NADA 095-143, June 15, 2021 TM-100D and TM-50D (oxytetracycline), NADA 008-804, June 15, 2021 Osurnia... DailyMed DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical gases, devices, cosmetics, dietary supplements, and medical foods. The NLM provides DailyMed to the public and does not accept advertisements. Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Code of Federal Regulations Title 21 - Food and Drug Administration (6) The requirement in § 201.80 (f) (2) to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the prescription drug labeling must be implemented no... Labeling and Label Approval | Food Safety and Inspection Service Collection of guidance information on label procedures with regards to label applications, types of approval, common labeling questions and answers and information on the responsibilities of FSIS and the establishments regarding label approvals. ... the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2 ... Drugs@FDA: FDA-Approved Drugs All Approvals and Tentative Approvals June 2022 This report includes approvals of NDAs, BLAs, ANDAs, and approved supplements to those applications, and tentative ANDA/NDA approvals during the... Drugs@FDA: FDA-Approved Drugs All Approvals and Tentative Approvals January 2022 This report includes approvals of NDAs, BLAs, ANDAs, and approved supplements to those applications, and tentative ANDA/NDA approvals during the...
FDA Drug Safety Podcast: FDA approves label changes for use of general ... Listen to an audio podcast of the April 27, 2017 Drug Safety Communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children. FDA is notifying the public that we have approved label changes previously announced in December 2016 regarding the use of general anesthetic and sedation medicines in pregnant women and children younger than 3 years. Drugs@FDA: FDA-Approved Drugs Original BLA/NDA approvals by CBER are not included in Drugs@FDA. This report does not include approved NDA or BLA supplements, approved ANDAs, or tentatively approved ANDAs/NDAs. Click on the Drug... FDA List of Authorized Generic Drugs | FDA To obtain approval of a generic drug, a company must submit an Abbreviated New Drug Application (ANDA) to FDA and prove that its product is the same as the brand-name drug in the ways described... What are the FDA Labeling Requirements for Cosmetic Products? The FDA regulates cosmetic labeling under the authority of both the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA). The FD&C Act was created in 1938, authorizing the FDA to oversee the safety of food, drugs, and cosmetics. However, it does not require that cosmetics receive FDA approval before ...
National Drug Code Directory | FDA Drug establishments are required to provide FDA with a current list of all drugs manufactured, prepared, propagated, compounded or processed for sale in the U.S. at their facilities. Drugs are...
FDA grants accelerated approval to dabrafenib in combination with ... on june 22, 2022, the food and drug administration granted accelerated approval to dabrafenib (tafinlar, novartis) in combination with trametinib (mekinist, novartis) for the treatment of adult and...
FDA Approves Label Extension for Evrysdi for Infants with Spinal ... SOUTH PLAINFIELD, N.J., May 31, 2022 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi ( risdiplam) to include infants under 2 months old with spinal muscular atrophy (SMA).
Galderma Receives FDA Approval for New SCULPTRA® Label, Offering More ... New label update allows for immediate use after reconstitution and more convenient administration of SCULPTRA ® with the optional addition of lidocaine for increased patient comfort; Fort Worth, Texas - December 13, 2021 - Galderma announced today the new label for SCULPTRA ® (injectable poly-L-lactic acid (PLLA)), the first and only FDA-approved PLLA facial injectable treatment that ...
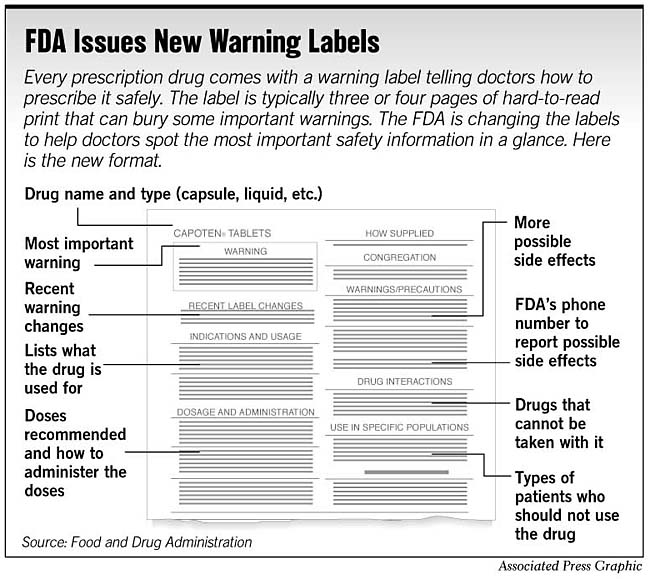
Introduction to the New Prescription Drug Labeling by the FDA A prescription drug product label (also known as a professional label, package insert, direction circular, and package circular) is a compilation of information about a product written by the...
Introduction to the New Prescription Drug Labeling by the FDA A separate section on patient-counseling information has been added in response to the requirement that all FDA-approved patient information be reprinted in or accompany drug product labeling.
FDA Approves Label Extension for Evrysdi® for Infants with Spinal ... PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi® (risdiplam) to include infants under 2 months ...
"Approved by FDA" Labeling Statement for Approved New Animal Drugs Introduction This is a message to remind you that one of the following statements (as applicable) must be included on your approved (A)NADA labeling (except representative [Blue Bird] labeling) by...
FDA Approves Label Extension for Evrysdi® for Infants with Spinal ... SOUTH PLAINFIELD, N.J., May 31, 2022 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi ® (risdiplam) to include infants under 2 months old with spinal muscular atrophy (SMA).
Drugs@FDA: FDA-Approved Drugs This report displays final approvals and tentative approvals of original and supplemental applications for the two weeks beginning on the earliest date listed below. Some approvals may be added to...
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...








Post a Comment for "40 fda approved drug labels"