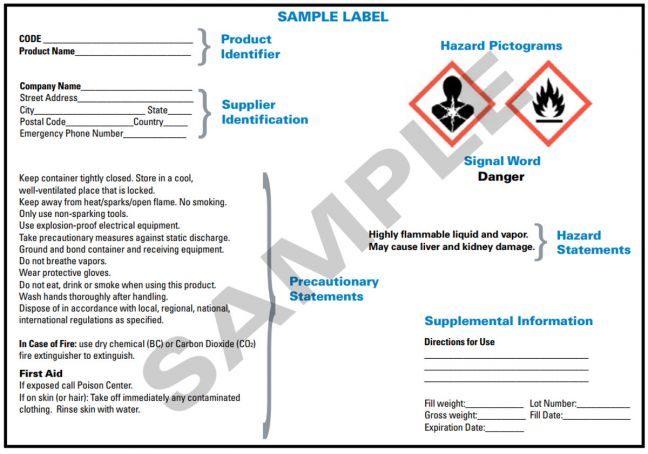
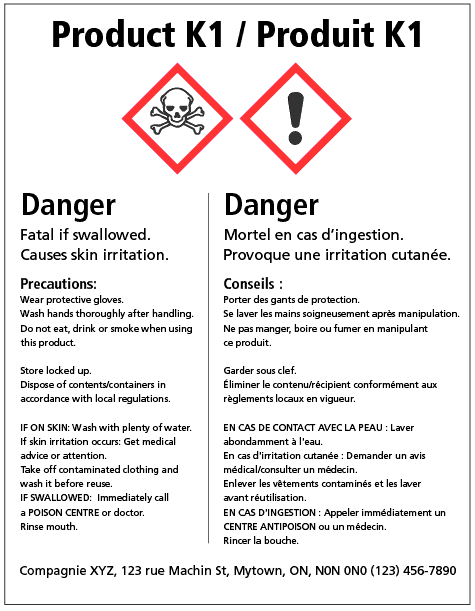
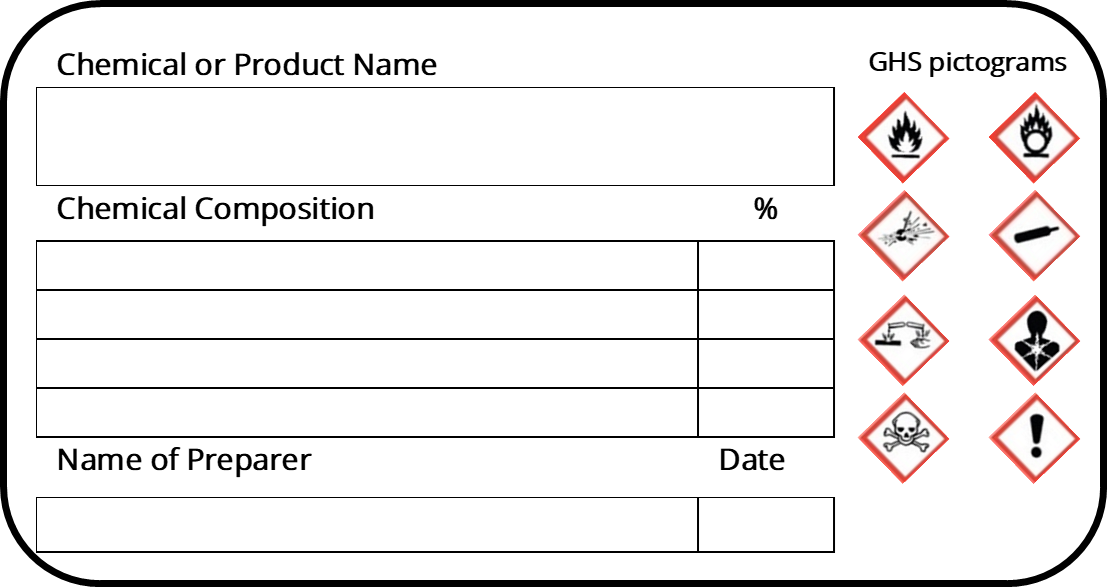
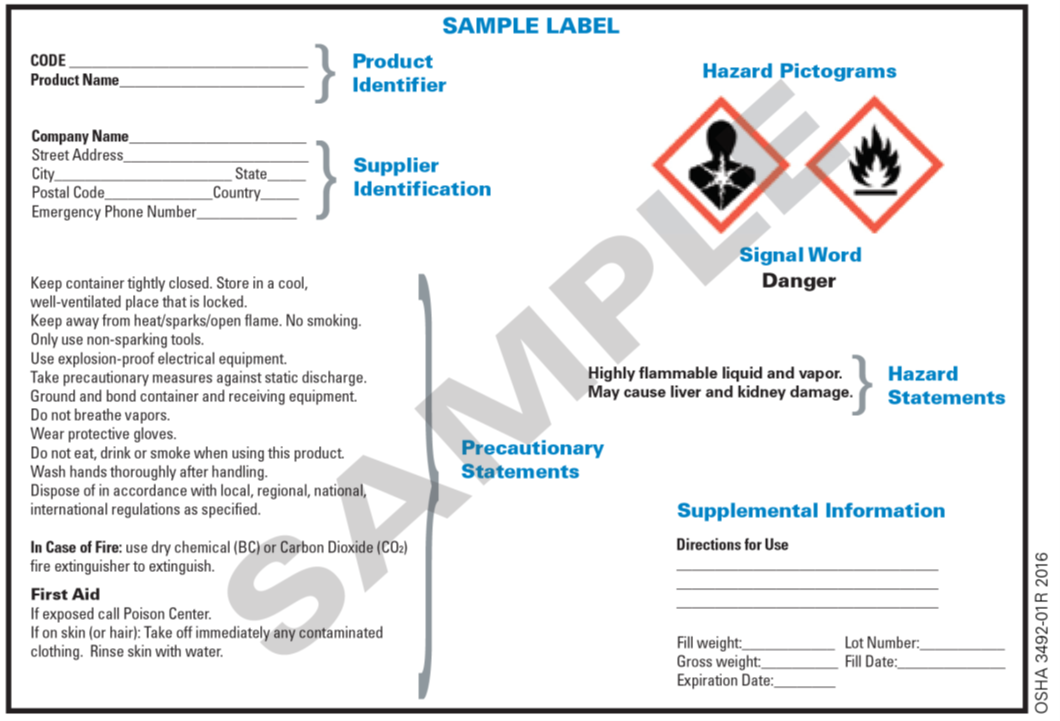
39 what must be included on chemical labels
› resources › acronyms-and-glossary-termsAcronyms and glossary terms | Therapeutic Goods ... A name for an ingredient, or a plant or other organism included in the formulation of a medicine, which is included in the list of TGA Approved Terminology for Medicines published by the Therapeutic Goods Administration. The list comprises three parts: Chemical substances AAN list; Herbal substances AAN list; and Biological substances AAN list. › pesticide-labels › pesticide-labelingPesticide Labeling Questions & Answers | US EPA Oct 13, 2022 · What other information must be included in these booklets? LC06-0068 The booklet is considered “labeling,” defined in part as: “all labels and all other written, printed, or graphic matter (A) accompanying the pesticide or device at any time; or (B) to which reference is made on the label or in literature accompanying the pesticide or ...
› Tags › SatelliteSatellite News and latest stories | The Jerusalem Post Mar 08, 2022 · The Jerusalem Post Customer Service Center can be contacted with any questions or requests: Telephone: *2421 * Extension 4 Jerusalem Post or 03-7619056 Fax: 03-5613699 E-mail: [email protected ...
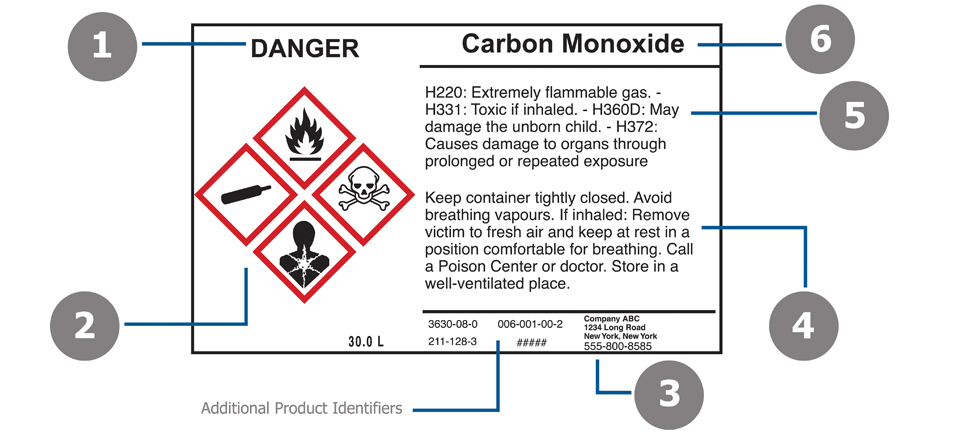
What must be included on chemical labels
› pet-food-labels-generalPet Food Labels - General | FDA If more than one ingredient is included in a "dinner" name, the combination of the named ingredients must total 25% of the product and be listed in the same order as found on the ingredient list. › sites › defaultBRIEF - Occupational Safety and Health Administration immediate recognition of the hazards. Labels must also provide instructions on how to handle the chemical so that chemical users are informed about how to protect themselves. The label provides information to the workers on the specific hazardous chemical. While labels provide important information for anyone who handles, uses, stores, and en.wikipedia.org › wiki › Packaging_and_labelingPackaging and labeling - Wikipedia Information transmission – Packages and labels communicate how to use, transport, recycle, or dispose of the package or product. With pharmaceuticals, food, medical, and chemical products, some types of information are required by government legislation. Some packages and labels also are used for track and trace purposes.
What must be included on chemical labels. › documents › 2018/12/21Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. en.wikipedia.org › wiki › Packaging_and_labelingPackaging and labeling - Wikipedia Information transmission – Packages and labels communicate how to use, transport, recycle, or dispose of the package or product. With pharmaceuticals, food, medical, and chemical products, some types of information are required by government legislation. Some packages and labels also are used for track and trace purposes. › sites › defaultBRIEF - Occupational Safety and Health Administration immediate recognition of the hazards. Labels must also provide instructions on how to handle the chemical so that chemical users are informed about how to protect themselves. The label provides information to the workers on the specific hazardous chemical. While labels provide important information for anyone who handles, uses, stores, and › pet-food-labels-generalPet Food Labels - General | FDA If more than one ingredient is included in a "dinner" name, the combination of the named ingredients must total 25% of the product and be listed in the same order as found on the ingredient list.
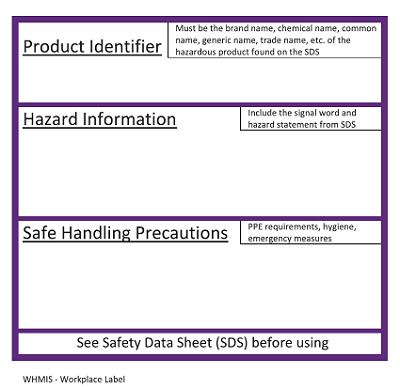



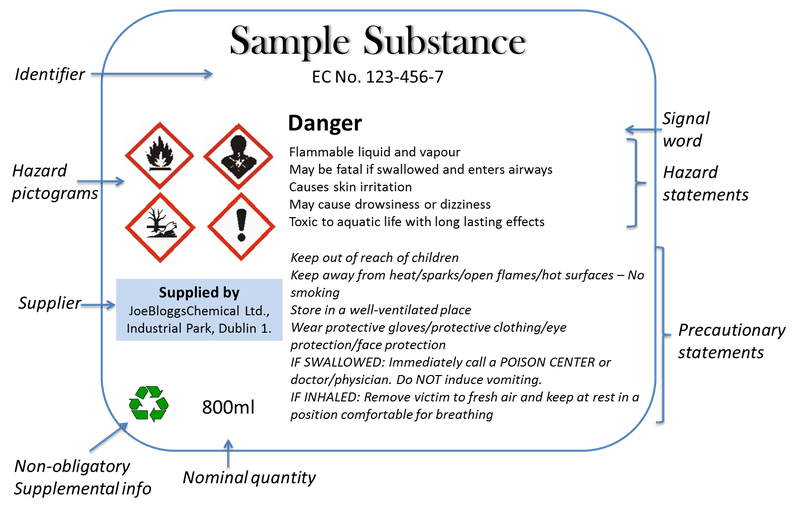




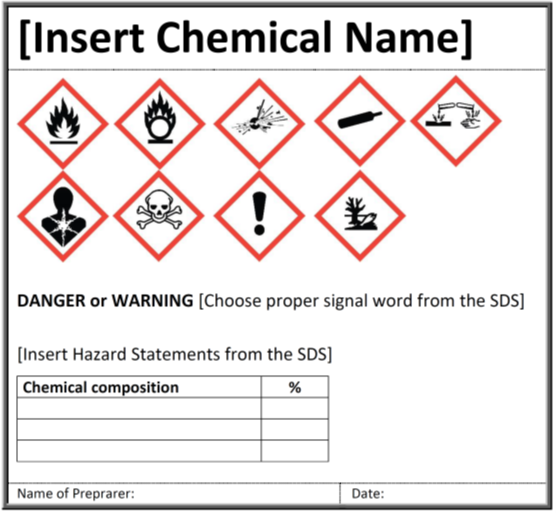















Post a Comment for "39 what must be included on chemical labels"